
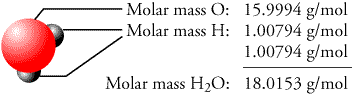
Dispersion forces are decisive when the difference is molar mass or. Molar Mass of Water Molar Mass of H2O (Water) (Our Other Math & Science Tools) Molar Weight of H2O (Water) Formula > Weight Theoretical Yield Percent Yield Uses the formula of a reactant to determine molar mass. Calculate the percentage of water in hydrate.Divide the molar mass of water by the molar mass of the hydrate, and multiply result by 100%.36.04g147.01g x 100%Percent water in hydrate is 24.52%. For example, water has London dispersion, dipole-dipole, and hydrogen bonds. This number may be useful to remember on the day of the test or while doing practice problems.*2. Add these values together to find the molar mass of the hydrate.Molar Mass Anhydrate + Molar Mass Water Molecules* = Molar Mass Hydrate* Tip: the molar mass of water for all hydrate calculations is 18.02g x number of water molecules. Do this for both the anhydrate and the water molecules. etc.Add up all the mass values and you have the value for molar mass. Find the molar mass of the hydrate (Calcium Chloride Dihydrate).Find the molar mass of water and the anhydrate (anhydrate + water = hydrate) add the molar mass values of each to find the molar mass of the hydrate.Molar Mass CaCl2: 110.98g+ Molar Mass H2O: 36.04g*Molar Mass CaCl2 * 2H2O: 147.01gFinding Molar Mass# atoms element A * atomic mass element A = Mass A# atoms element B * atomic mass element B = Mass B. MM = Molar Mass Water (H 2O) Example ProblemĢ X (MM of hydrogen) + MM of Oxygen = 2 x (1.01g) + 16.0 = 18.What is the theoretical percentage of water of hydration in cacl2-2h20?ġ. Moreover, this means that 1 mole of NH 3 is equal to 17.04 grams of NH 3. Atomic mass is the number of grams per mole of the element. From the periodic table we see the atomic weight of hydrogen is 1.0079 and the atomic weight of oxygen is 15.9994.

#Molar mass of water plus
A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. The mass of hydrogen is 1.008 g/mol and the mass of oxygen is 16.00 g/mol, so the mass of a mole of water can be calculated as. Water (H 2 O) is made from 2 atoms of hydrogen and 1 atom of oxygen. For ammonia, we should get a molar mass of 17.04 grams. Do this by adding up the mass of hydrogen atoms and oxygen atoms in a mole of water by looking up the atomic mass of hydrogen and oxygen on the periodic table. The last and final step is to add up the two products we got from multiplying. So, now we will multiply these numbers by their corresponding atomic masses. According to the molecular formula (NH 3), there is one nitrogen and three hydrogens present. The second step is to determine how much of each element is present in the compound. For example, if we are trying calculate for ammonia (NH 3), then we need to find the atomic masses for nitrogen and hydrogen. The atomic mass is equal to the atomic number which is listed below the element symbol. The first step for calculating molar mass is to identify all the elements in a given molecule and write their atomic masses using the periodic table. Molecular weight is represented by the same number in all unit systems. In the SI system the unit of M is kg/kmol and in the English system the unit is lb/lbmol, while in the cgs system the unit of M is g/mol. Let’s look at some examples of calculating this value for some different molecules. The molecular weight of a substance, also called the molar mass, M, is the mass of 1 mole of that substance, given in M gram. It should be noted that this number is an average and therefore may vary due to isotopic elements. Molar mass can be calculated by using the periodic table and following three simple steps. The units of molar mass is grams per mole, often abbreviated g/mol. That’s a heck of a lot of molecules, which is why the molar mass of table salt (sodium chloride) is a respectable 58.44 grams per mole, quite a large handful. Both definitions will give you the same result, they are just in different units.Ī “mole” of a substance is defined as 6.022 x 10 23 atoms or molecules of that substance. Another definition of molar mass, is the sum of the atomic weights of the atoms that make up a molecule. The definition of molar mass is simply the number of grams that one mole of a substance weighs.
#Molar mass of water how to
How to Write Electron Shell Configurations.Quantifying Protons, Neutrons, and Electrons.If you enjoy this article, be sure to check out our other tutorials linked below. You will learn how to find and calculate the molar mass for elements and molecules. In this tutorial, we will explain “what is molar mass”.


 0 kommentar(er)
0 kommentar(er)
